Introduction
Chimeric Antigen Receptor T-Cell (CAR-T cell) Therapy faces challenges related to low potency and poor in vivo persistence. Incorporating cytokine signals, such as interleukin-12 (IL-12), has emerged as a promising strategy to enhance CAR-T cell functionality due to its potent immunostimulatory effects. However, clinical application of IL-12 is hindered by pleiotropic toxicity and limited bioavailability. Therefore, alternative agents or delivery systems are desired to exploit the benefits of IL-12 safely. This study investigated the therapeutic potential of a novel IL-12 delivery system utilizing extracellular vesicles (EVs) modified with the target antigen CD19 to specifically deliver IL-12 to CD19 CAR-T cells, thus augmenting their anti-tumor effects.
Method
IL-12-anchored extracellular vesicles (IL-12 EVs) were generated from engineered 293T cells expressing IL-12 on the cell membrane via GPI structure. Additionally, EVs co-expressing IL-12 and CD19 (CD19/IL-12 EVs) were produced from 293T cells co-expressing CD19 and IL-12. The physicochemical characteristics of the EVs were analyzed using nanoparticle tracking analysis (NTA), transmission electron microscopy (TEM), and western blot (WB). Binding and uptake efficiency of the EVs with CD19 CAR-T cells were validated using flow cytometry and imaging flow cytometry. In vitro experiments were conducted to investigate the effects of the EVs on the cytotoxic function, degranulation, cytokine secretion, and proliferation of CD19 CAR-T cells. Furthermore, the therapeutic efficacy and safety of CD19 CAR-T cells combined with intratumoral injection of IL-12 EVs were evaluated in a mouse model of Raji lymphoma xenograft.
Result
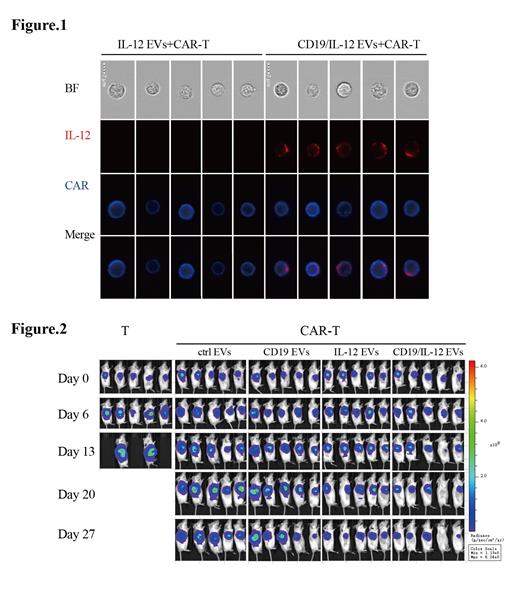
HEK293T cell-derived, IL-12-bearing EVs were successfully prepared. IL-12 on the surface of EVs was quantified by enzyme-linked immunosorbent assay. IL-12 EVs promoted interferon-γ secretion of co-cultured CAR-T cells in a dose-dependent manner, reaching a peak at a concentration of about 1 ng/mL of IL-12 EVs. At this concentration, IL-12 EVs significantly enhanced CAR-T cell cytotoxicity while recombinant human interleukin-12 not. Co-expressing CD19 and IL-12 EVs were prepared and visualized by imaging flow cytometry, showing the presence of both IL-12 and CD19 on the surface of each individual EV. CD19/IL-12 EVs exhibited stronger tropism towards anti-CD19 CAR-T cells than IL-12 EVs (Figure 1). Similarly, CD19/IL-12 EVs could promote CAR-T cells with increased cytokine secretion and improved tumor-killing ability. In a Raji subcutaneous lymphoma xenograft mouse model, intratumoral injection of CD19/IL-12 EVs observably promoted CAR-T cell function, achieved superior proliferation and enhanced anti-tumor effects without systemic toxicity (Figure 2).
Conclusion
This study demonstrated that surface modification of EVs with target antigens empowers EVs with improved CAR-T cell targeting, creating an effective delivery platform for targeted delivery of bioactive molecules, such as IL-12, to CAR-T cells, thereby enhancing immunotherapeutic efficacy.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal